Chemical reaction is a process in which molecules break up into atoms and atoms rearrange to form new molecules. The chemical reaction took place in a very short time, within picoseconds. In the past two decades, scientists have used ultrafast lasers to capture fast images of reactions, but they can't capture all of them. Now, using supercooled chemistry, researchers can first understand the exact situation of the chemical reaction process.

Scientists in the United States published a paper in the latest issue of science, saying that at a temperature millions of times colder than that of interstellar space, they made two supercooled molecules have the coldest chemical reaction in the universe so far, forming the coldest bond in the history of molecular coupling, and let us witness a chemical reaction from beginning to end for the first time in history.
Researchers at Harvard University said that at 500 nackelvin (only a few millionths of a degree Celsius higher than absolute zero), the speed of the molecules dropped to a very low level, and they saw a scene that no one had ever seen before: the moment when two molecules meet to form two new molecules.
In previous studies, the team used ultra-low temperatures to make molecules from atoms that would otherwise not have reacted. At very low temperatures, atoms and molecules decelerate to the lowest possible energy state, allowing researchers to most precisely manipulate molecular interactions. But even so, they can only see the beginning of the reaction: two molecules enter, and what happens to them is a "reaction black hole";.
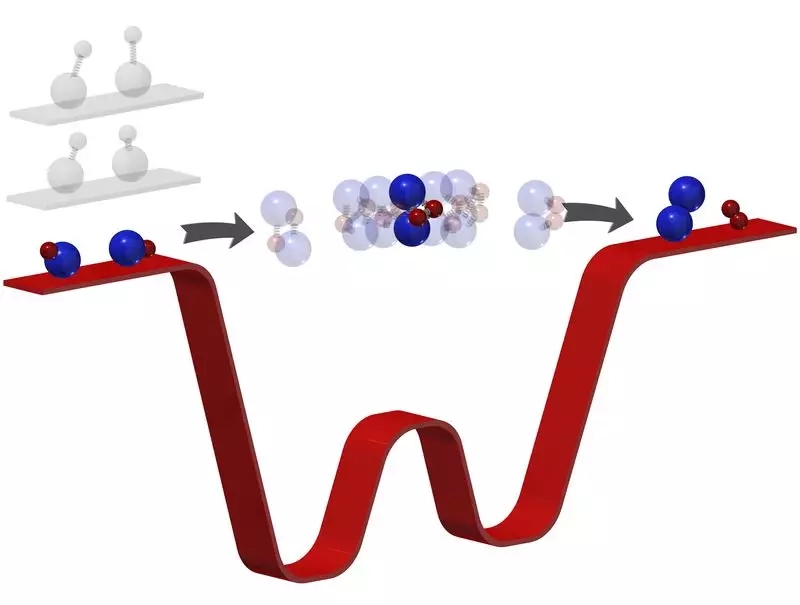
Because chemical reactions take place in picoseconds, even the most advanced technology can not capture such a short picture. Over the past 20 years, scientists have been using ultrafast lasers to capture the full picture of the reaction. &"Most of the time, you just see the reactant disappear, and then the product appears, but we don't know what happened in the middle. ”
Now, the team's supercooled temperature allowed them to see what happened during the reaction for the first time. When they let two rubidium potassium react, the supercooling temperature forces the molecule to stay for several microseconds in the intermediate stage, which is millions of times longer than usual, enough for them to study the process of bond breaking and Formation & mdash; & mdash; that is, how one molecule becomes another.
With this new method, they can verify various theories of predicting the occurrence of reactions, and can put forward new theories to more accurately predict the occurrence of other chemical reactions (even in the quantum field). In addition, with the help of the new reaction platform, they can manipulate the reaction objects, excite them before the reaction, to see the impact of increased energy on the reaction results, or even manipulate the reaction process.
Chemical reactions are actually responsible for everything: breathing, cooking, digesting, creating energy, drugs, soap and other household products. So understanding how they work at a fundamental level can help researchers design combinations that have never been seen in the world. With almost unlimited new combinations, these new molecules will have endless applications, from more efficient energy production to new materials.
The team is already exploring what they can learn on the supercooled test bed. Next, for example, they can manipulate the reactants and excite them before they react to see how their increased energy affects the results. Or, they may even affect the course of the reaction, touching one molecule or another.
Source: Science and technology daily, principle

